TMS Efficacy
Efficacy in Depression
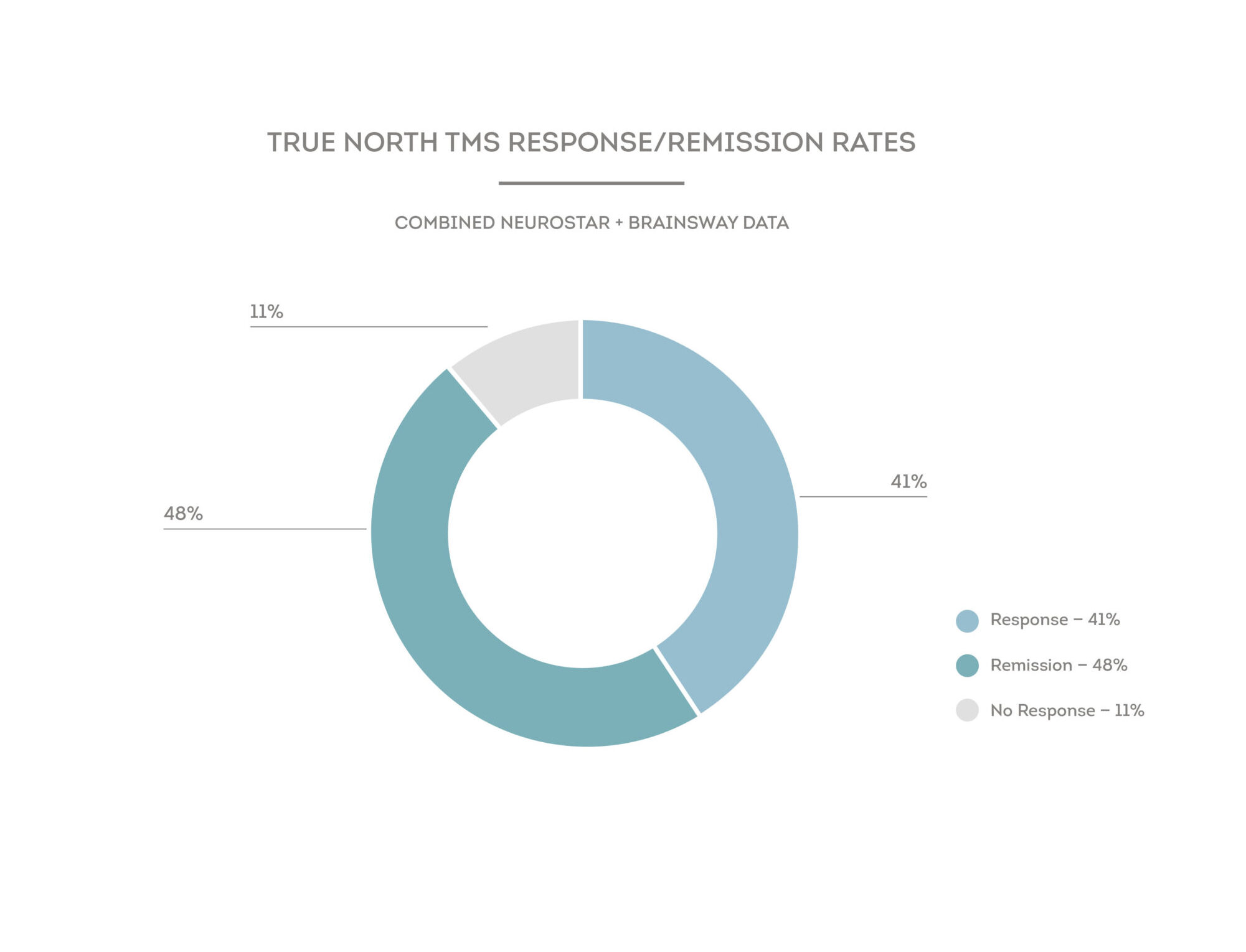
Clinical studies have shown that one in two patients treated with TMS had a 50% reduction in their symptoms, and after six weeks of treatment, one-third (33%) of patients had no symptoms of their depression or were in “remission.” While there has not been a head-to-head trial that compares TMS to antidepressants, a landmark study (called STAR*D) explored the efficacy of medication to treat patients suffering from MDD and less than 15% achieved “remission.”
It is also important to note that TMS treatment may not eliminate the need to continue taking antidepressant medication, but it could reduce the amount of medication needed by the patient and, thereby, minimize the side effects associated with them.
Clinical studies have shown that one in two patients treated with TMS had a 50% reduction in their symptoms, and after six weeks of treatment, one-third (33%) of patients had no symptoms of their depression or were in “remission.” While there has not been a head-to-head trial that compares TMS to antidepressants, a landmark study (called STAR*D) explored the efficacy of medication to treat patients suffering from MDD and less than 15% achieved “remission.”
It is also important to note that TMS treatment may not eliminate the need to continue taking antidepressant medication, but it could reduce the amount of medication needed by the patient and, thereby, minimize the side effects associated with them.
Efficacy in Depression
Clinical studies have shown that one in two patients treated with TMS had a 50% reduction in their symptoms, and after six weeks of treatment, one-third (33%) of patients had no symptoms of their depression or were in “remission.” While there has not been a head-to-head trial that compares TMS to antidepressants, a landmark study (called STAR*D) explored the efficacy of medication to treat patients suffering from MDD and less than 15% achieved “remission.”
It is also important to note that TMS treatment may not eliminate the need to continue taking antidepressant medication, but it could reduce the amount of medication needed by the patient and, thereby, minimize the side effects associated with them.
Clinical studies have shown that one in two patients treated with TMS had a 50% reduction in their symptoms, and after six weeks of treatment, one-third (33%) of patients had no symptoms of their depression or were in “remission.” While there has not been a head-to-head trial that compares TMS to antidepressants, a landmark study (called STAR*D) explored the efficacy of medication to treat patients suffering from MDD and less than 15% achieved “remission.”
It is also important to note that TMS treatment may not eliminate the need to continue taking antidepressant medication, but it could reduce the amount of medication needed by the patient and, thereby, minimize the side effects associated with them.
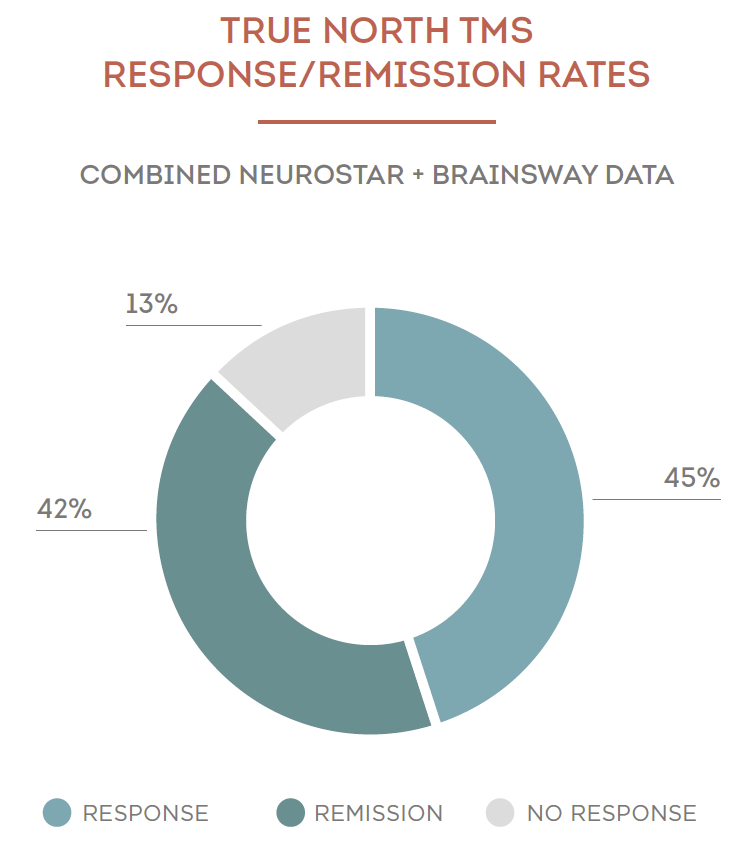
89% of patients treated at True North TMS at Willow Medical have shown significant improvement in their depression after TMS treatment.
87% of patients treated at True North TMS at Willow Medical have shown significant improvement in their depression after TMS treatment.
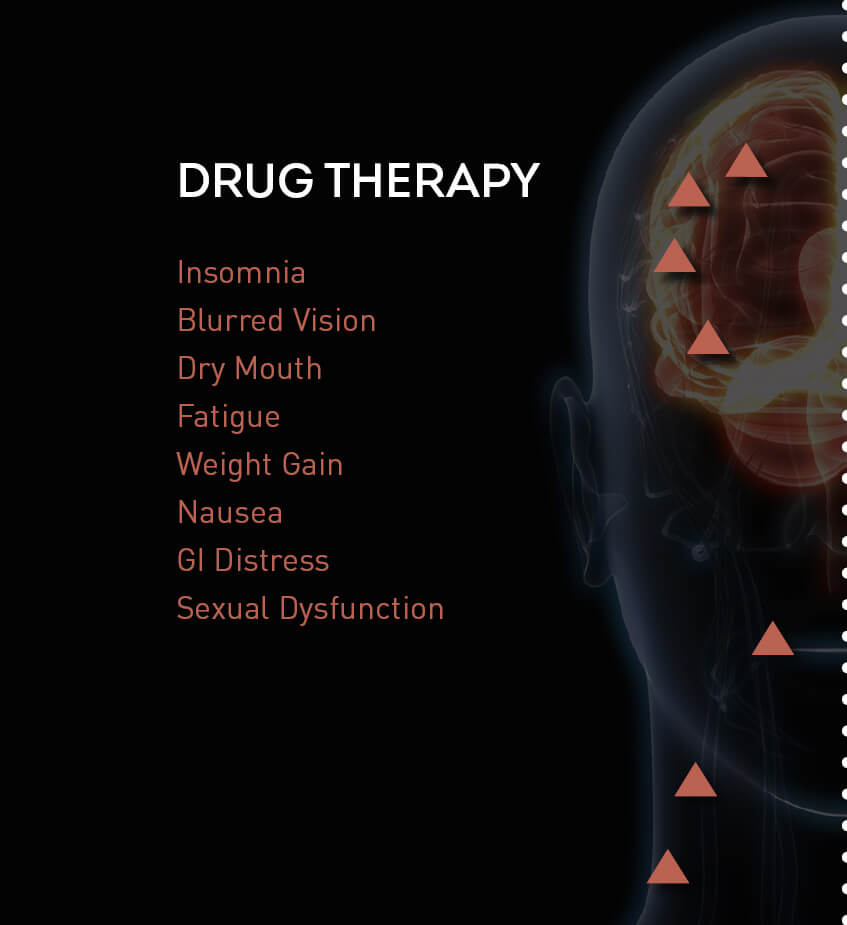
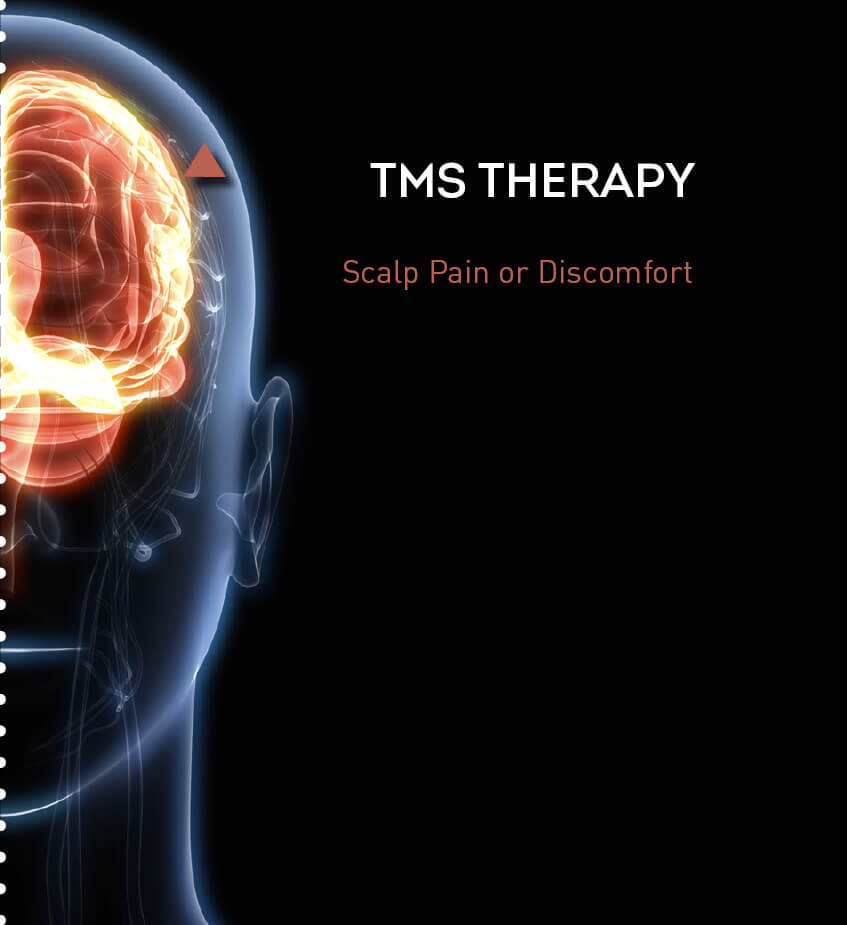
TMS Safety and Side Effects
- No systemic side effects
- No weight gain
- No sexual dysfunction
- No sedation
- No nausea
- No dry mouth
- No adverse effects on concentration or memory
- No drug interactions
- TMS Therapy should not be used in patients with implanted metallic devices or non-removable metallic objects in or around the head. This does not include metallic fillings in teeth.
- TMS Therapy should not be used in patients with implants controlled by physiological signals. This includes pacemakers, implantable cardioverter defibrillators (ICDs), and vagus nerve stimulators (VNS).
- TMS Therapy should be avoided in patients with a known seizure disorder or who has a medical condition that puts them at increased risk for having a seizure.
TMS Safety and Side Effects
- No systemic side effects
- No weight gain
- No sexual dysfunction
- No sedation
- No nausea
- No dry mouth
- No adverse effects on concentration or memory
- No drug interactions
- TMS Therapy should not be used in patients with implanted metallic devices or non-removable metallic objects in or around the head. This does not include metallic fillings in teeth.
- TMS Therapy should not be used in patients with implants controlled by physiological signals. This includes pacemakers, implantable cardioverter defibrillators (ICDs), and vagus nerve stimulators (VNS).
- TMS Therapy should be avoided in patients with a known seizure disorder or who has a medical condition that puts them at increased risk for having a seizure.
POTENTIAL SIDE EFFECTS