Our TMS Technologies
True North TMS is the leading provider of TMS therapy in Anchorage, Alaska. We utilize state-of-the art-technology to deliver cutting edge depression treatment to patients.
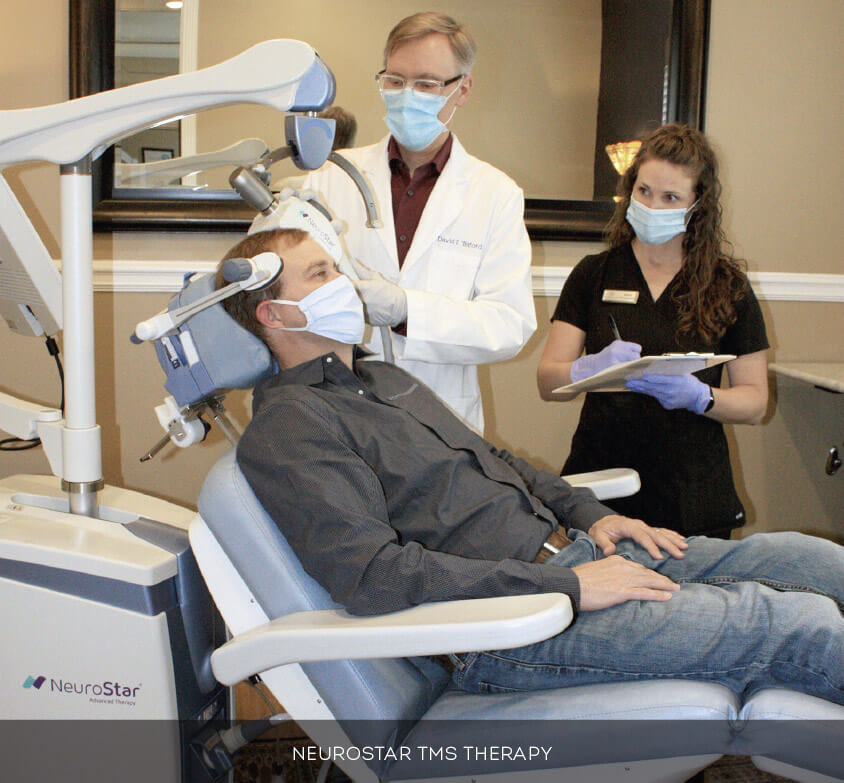
NeuroStar Advanced Therapy (rTMS) is an FDA-cleared, non-drug treatment for patients with MDD who have failed to receive satisfactory improvement from prior antidepressant medication in the current episode. Proven safe and effective, NeuroStar achieves results without the side effects often associated with antidepressant medications.
The NeuroStar TMS system uses a highly focused pulsed magnetic field to stimulate cortical neurons, stimulating blood flow, glucose metabolism and neurotransmitter release. A high-performance contoured coil delivers patented precision therapy with use of contact sensing technology and a 3-D laser positioning system.
BrainsWay’s Deep TMS therapy is an effective, safe, and FDA-cleared treatment for depression and OCD in patients who failed to respond to antidepressant medications and psychotherapy. BrainsWay’s therapy is non-invasive, does not cause systemic side effects, and does not require hospitalization or anesthesia.
BrainsWay’s Deep TMS flagship technology is based on the H-Coil, which features a novel patented structure designed to maximize electrical stimulation of deep brain regions. Deep TMS works by utilizing a magnetic field that manages to directly reach wider and deeper brain regions, regulating the neural activity of brain structures related to depression – specifically the dorsolateral prefrontal cortex (DLPFC). The coil creates a temporary magnetic field stimulating the underactive areas of the brain that regulate mood.
NeuroStar Advanced Therapy (rTMS) is an FDA-cleared, non-drug treatment for patients with MDD who have failed to receive satisfactory improvement from prior antidepressant medication in the current episode. Proven safe and effective, NeuroStar achieves results without the side effects often associated with antidepressant medications.
The NeuroStar TMS system uses a highly focused pulsed magnetic field to stimulate cortical neurons, stimulating blood flow, glucose metabolism and neurotransmitter release. A high-performance contoured coil delivers patented precision therapy with use of contact sensing technology and a 3-D laser positioning system.
During a NeuroStar Advanced Therapy (rTMS):
- You will recline comfortably in the treatment chair
- A small, curved magnetic coil will be positioned lightly on your head
- NeuroStar delivers focused magnetic stimulation directly to target areas of the brain
- You’ll hear a clicking sound and feel a tapping sensation in your head
- After treatment, you can resume normal activities
- Because there are no effects on alertness or memory, you can drive yourself to and from treatment sessions
- In-office treatments takes 19 min to deliver
NeuroStar Advanced Therapy (TMS) is safe and easy to tolerate. More than 4 million treatments have been performed with NeuroStar, further proving its safety and tolerability record. NeuroStar TMS therapy is backed by the largest clinical data set including a study demonstrating durability in response rates over a 12-month period.rgb(102, 153, 153)
Visit neurostar.com for indications for use and safety information.
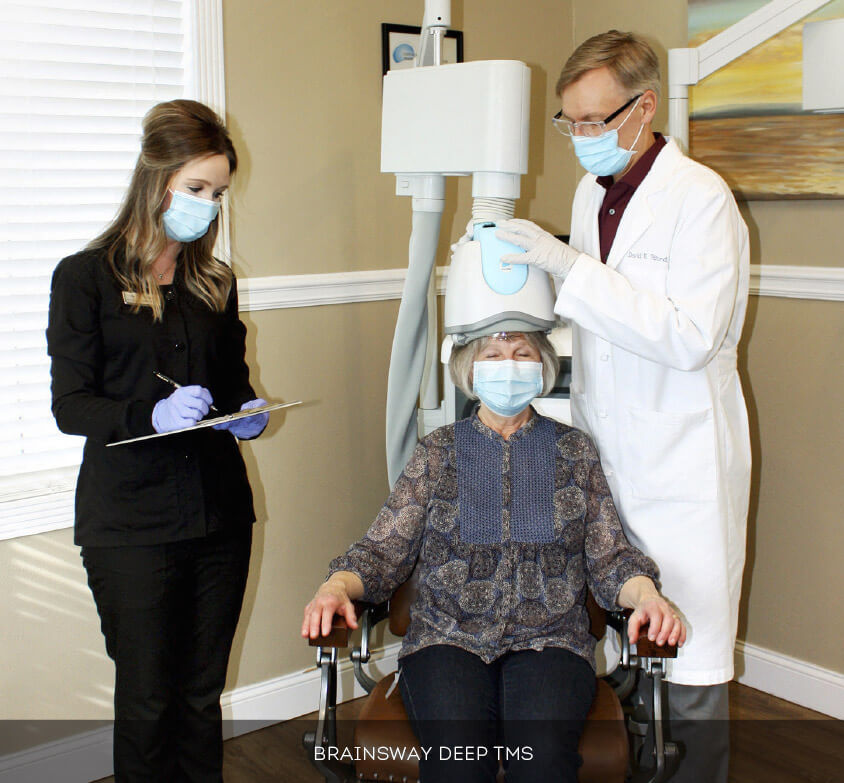
BrainsWay’s Deep TMS therapy is an effective, safe, and FDA-cleared treatment for depression and OCD in patients who failed to respond to antidepressant medications and psychotherapy. BrainsWay’s therapy is non-invasive, does not cause systemic side effects, and does not require hospitalization or anesthesia.
BrainsWay’s Deep TMS flagship technology is based on the H-Coil, which features a novel patented structure designed to maximize electrical stimulation of deep brain regions. Deep TMS works by utilizing a magnetic field that manages to directly reach wider and deeper brain regions, regulating the neural activity of brain structures related to depression – specifically the dorsolateral prefrontal cortex (DLPFC). The coil creates a temporary magnetic field stimulating the underactive areas of the brain that regulate mood.
During a Brainsway Deep TMS Session:- You are comfortably seated on a chair
- A cushioned helmet is placed over your head
- The helmet uses magnetic fields to stimulate deeper and broader regions of the brain
- The coil design itself includes a flexible base that can be adjusted to fit over different head shapes and sizes
- You will hear a repetitive tapping sound coming from within the helmet *
- Because there are no effects on alertness or memory, you can drive yourself to and from treatment sessions
- In-office treatments are 20 minutes long
BrainsWay’s Deep TMS has been tested in over 60 clinical trials worldwide. Numerous clinical studies in reputable peer-reviewed journals have found that BrainsWay’s Deep TMS therapy is safe and successful in alleviating symptoms of mental health conditions and offering patients relief. In August of 2018, BrainsWay became the first TMS device FDA-cleared for treatment of OCD using Deep TMS therapy.
FDA Cleared TMS Indications
BrainsWay’s treatment now offers an FDA-cleared, effective, safe and non-invasive treatment that uses Deep TMS for smoking cessation. The treatment performs magnetic stimulation of brain structures and networks related to this addiction and brings significant
improvement to patients.